| Kinase Assay: |
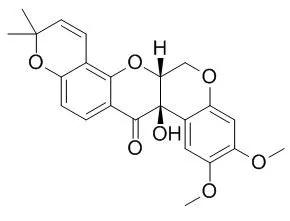
| Cancer Lett. 2010 Jul 1;293(1):23-30. | | Tephrosin induces internalization and degradation of EGFR and ErbB2 in HT-29 human colon cancer cells.[Pubmed: 20056314 ] | Inactivation of epidermal growth factor receptor (EGFR) family members are prime targets for cancer therapy.
METHODS AND RESULTS:
Here, we show that Tephrosin, a natural rotenoid which has potent antitumor activities, induced internalization of EGFR and ErbB2, and thereby induced degradation of the receptors. Treatment of HT-29 cells with Tephrosin inhibited both the ligand-induced and constitutive phosphorylation of EGFR, ErbB2 and ErbB3, and concomitantly suppressed the activation of the downstream signaling molecules such as Akt and Erk1/2 mitogen-activated protein kinase (MAPK). Tephrosin caused internalization of EGFR and ErbB2 into vehicles, which resulted in degradation of the receptors. This degradation was blocked by the lysosomal inhibitor, chloroquine. We also showed that Tephrosin induced apoptosis. Tephrosin did not induce the proteolytic processing of caspase-3 and poly(ADP-ribose) polymerase (PARP), but did nuclear translocation of apoptosis-inducing factor (AIF), suggesting that Tephrosin may induce caspase-independent apoptosis.
CONCLUSIONS:
These findings provide the first evidence that Tephrosin could exert antitumor effects by inducing internalization and degradation of inactivated EGFR and ErbB2 in human colon cancer cells. |
|
| Cell Research: |
| J Asian Nat Prod Res. 2010 Nov;12(11):992-1000. | | Tephrosin-induced autophagic cell death in A549 non-small cell lung cancer cells.[Pubmed: 21061222] | Anticancer effect of Tephrosin (1) has been documented; however, the molecular mechanisms underlying the cytotoxicity of Tephrosin in cancer cells remain unclear.
METHODS AND RESULTS:
In the present paper, the proliferation inhibition rate of several cancer cells was tested using the MTT assay; cell cycle, reactive oxygen species (ROS), and mitochondrial membrane potential (MMP) were determined by flow cytometry; poly(ADP-ribose) polymerase (PARP) cleavage and heat shock protein 90 (Hsp90) expression were evaluated by Western blotting; autophagy was examined by confocal microscopy and light chain 3 (LC3) conversion assay. The results showed that exposure of the cells to Tephrosin induced significant proliferation inhibition in a dose-dependent manner, especially on A549 with G(2)/M being arrested. Tephrosin was not found to induce cell apoptosis as PARP cleavage was not detected after 24 h treatment, but the formation of acidic vesicular organelle of autophagy character was found, and autophagy was further confirmed by the increase in the ratio of LC3-II to LC3-I. It was observed that Tephrosin induced ROS generation and Hsp90 expression inhibition.
CONCLUSIONS:
These results indicate that Tephrosin induces A549 cancer cell death via the autophagy pathway, and the roles of ROS generation and Hsp90 expression inhibition in this process need further study in the future. | | Cancer Biol Ther. 2011 Dec 1;12(11):989-96. | | The combination of tephrosin with 2-deoxy-D-glucose enhances the cytotoxicity via accelerating ATP depletion and blunting autophagy in human cancer cells.[Pubmed: 22123175] | 2-Deoxy-D-glucose (2-DG), a synthetic glucose analog that acts as a glycolytic inhibitor, is currently under clinical evaluation for targeting tumor cells. Tephrosin (TSN), a plant rotenoid, is known as an anticancer agent.
METHODS AND RESULTS:
In this study, we describe that the addition of Tephrosin to 2-DG enhanced the cytotoxic activity of 2-DG against various types of cancer cells by accelerating ATP depletion and blunting autophagy. Tephrosin increased the sensitivity of cancer cells to the cytotoxic effect of 2-DG. The combination of Tephrosin and 2-DG induced acceleration of intracellular ATP depletion and the drastic activation of AMP-activated protein kinase (AMPK), which resulted in the inactivation of the mammalian target of rapamycin (mTOR) pathway. Of particular interest, Tephrosin suppressed 2-DG-induced autophagy, a cell survival process in response to nutrient deprivation. We also showed that Tephrosin inhibited 2-DG-induced activation of elongation factor-2 kinase (eEF-2K), which has been known to regulate 2-DG-induced autophagy. Inhibition of eEF-2K by RNA interference blunted 2-DG-induced autophagy and increased the sensitivity of cancer cells to the cytotoxic effect of 2-DG. The addition of Tephrosin to 2-DG, however, did not enhance the cytotoxic activity of 2-DG by knockdown of eEF-2K, suggesting that inhibition of eEF-2K by tephrsoin could be a critical role in the potentiating effect of Tephrosin on the cytotoxicity of 2-DG. Furthermore, we showed that the blunted autophagy and enhanced cytotoxicity of 2-DG was accompanied by the augmentation of apoptosis.
CONCLUSIONS:
These results show that Tephrosin may be valuable for augmenting the therapeutic efficacy of 2-DG. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)