METHODS AND RESULTS:
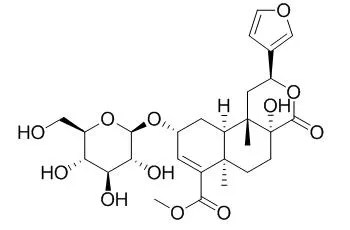
Three new clerodane diterpene glycosides, tinospinosides A (1), B (2), and C (3) were isolated from the roots of Tinospora sagittata (Oliv.) Gagnep. Their structures were determined to be (2 S,4a R,6a R,9 R,10a S,10b S)-2-(3-furanyl)-9-( β-D-glucopyranosyloxy)-1,4,4a,5,6,6a,9,10,10a,10b-decahydro-6a,10b-dimethyl-4-oxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (1), (2 S,4a S,6a R,9 R,10a R,10b S)-2-(3-furanyl)-9-( β-D-glucopyranosyloxy)-1,4,4a,5,6,6a,9,10,10a,10b-decahydro-4a-hydroxyl-6a,10b-dimethyl-4-oxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (2) and (2 S,4a R,6a R,9 R,10a R,10b S)-2-(3-furanyl)-9-( β-D-glucopyranosyloxy)-1,4,4a,5,6,6a,9,10,10a,10b-decahydro-4a-hydroxyl-6a,10b-dimethyl-4-oxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3), by various spectroscopic analyses, chemical reactions, and computer-assisted calculations.
The inhibitory activities of NO production by these compounds and their chemical derivatives in lipopolysaccharide and TNF γ-activated macrophage-like cell line J774.1 were tested.
CONCLUSIONS:
Tinospin A, 12- EPI-tinospin A, tinospinoside B, and Tinospinoside C showed inhibitory activities of NO production with the IC(50) values of 162, 182, 290, and 218 μM, respectively. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)