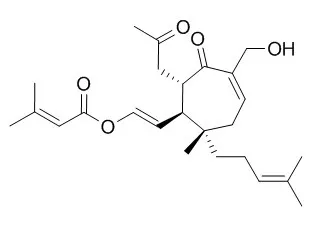
| Structure Identification: |
| J Nat Prod. 1999 Feb;62(2):337-9. | | Chemical conversion of vibsanin C to vibsanin E and structure of 3-hydroxyvibsanin E from viburnum awabuki.[Pubmed: 10075780] |
METHODS AND RESULTS:
Vibsanin E (4), a tricyclic vibsane-type diterpene, has been prepared in 50% yield from Vibsanin C (2), a seven-membered ring vibsane-type diterpene by reaction with BF3.OEt2 at -78 degrees C. This chemical correlation not only established structure, including absolute configurations, but also has demonstrated a possible biosynthetic route to 4 via 2 derived from vibsanin B (1).
CONCLUSIONS:
The structure of 3-hydroxyvibsanin E (5), another example of a tricyclic seven-membered ring vibsane, isolated from the leaves of Viburnumawabuki, has been established by extensive analyses of 2D NMR data and comparison of its spectral data with those of 4. | | J Nat Prod. 2004 Jan;67(1):74-7. | | Vibsane diterpenoids from the leaves and flowers of Viburnum odoratissimum.[Pubmed: 14738390 ] |
METHODS AND RESULTS:
In addition to the five known compounds 5-epi-vibsanin H, vibsanins C, H, and G, and aldovibsanin B, four new diterpenes, 5-epi-vibsanin G (1), 18-O-methylvibsanin G (2), vibsanin M (3), and aldoVibsanin C (4), were isolated from an acetone extract of the leaves and flowers of Viburnum odoratissimum by bioassay-directed fractionation. In addition, two acetyl derivatives 5 and 6 were obtained from the naturally occurring diterpenes.
CONCLUSIONS:
The structures of the new compounds were established on the basis of NMR spectral analysis, including COSY, HMQC, HMBC, and NOESY correlations. The compounds were evaluated for cytotoxicity against human nasopharyngeal carcinoma (HONE-1) tumor cells and human gastric cancer (NUGC-3) cells. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)