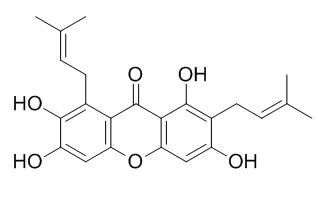
| Description: |
gamma-Mangostin is a dual agonist that activates both PPARδ and PPARα, is also a novel competitive antagonist for the 5-HT2A receptors in vascular smooth muscles and platelets.gamma-Mangostin has free radical scavenging activity, and antiproliferative and apoptotic activity in HepG2 cells, and exhibits antihypertensive, anti-inflammatory, analgesic effects. gamma-Mangostin could as a preventive agent of the metabolic syndrome, and could serve as a micronutrient for colon cancer prevention. |
| In vitro: |
| BMC Complement Altern Med. 2013 Jul 19;13:182. | | Antileptospiral activity of xanthones from Garcinia mangostana and synergy of gamma-mangostin with penicillin G.[Pubmed: 23866810] | Leptospirosis, one of the most widespread zoonotic infectious diseases worldwide, is caused by spirochetes bacteria of the genus Leptospira. The present study examined inhibitory activity of purified xanthones and crude extracts from Garcinia mangostana against both non-pathogenic and pathogenic leptospira. Synergy between gamma-Mangostinand penicillin G against leptospires was also determined.
METHODS AND RESULTS:
Minimal inhibitory concentrations (MIC) of crude extracts and purified xanthones from G. mangostana and penicillin G for a non-pathogenic (L. biflexa serovar Patoc) and pathogenic (L. interrogans serovar Bataviae, Autumnalis, Javanica and Saigon) leptospires were determined by using broth microdilution method and alamar blue. The synergy was evaluated by calculating the fractional inhibitory concentration (FIC) index.
The results of broth microdilution test demonstrated that the crude extract and purified xanthones from mangosteen possessed antileptospiral activities. The crude extracts were active against all five serovars of test leptospira with MICs ranging from 200 to ≥ 800 μg/ml. Among the crude extracts and purified xanthones, garcinone C was the most active compound against both of pathogenic (MIC =100 μg/ml) and non-pathogenic leptospira (MIC = 200 μg/ml). However, these MIC values were higher than those of traditional antibiotics. Combinations of gamma-Mangostin with penicillin G generated synergistic effect against L. interrogans serovars Bataviae, Autumnalis and Javanica (FIC = 0.52, 0.50, and 0.04, respectively) and no interaction against L. biflexa serovar Patoc (FIC =0.75). However, antagonistic activity (FIC = 4.03) was observed in L. interrogans serovar Saigon.
CONCLUSIONS:
Crude extracts and purified xanthones from fruit pericarp of G. mangostana with significant antibacterial activity may be used to control leptospirosis. The combination of xanthone with antibiotic enhances the antileptospiral efficacy.
| | J Pharm Pharmacol. 2013 Sep;65(9):1419-28. | | Antitumour and free radical scavenging effects of γ-mangostin isolated from Garcinia mangostana pericarps against hepatocellular carcinoma cell.[Pubmed: 23927480] | Liver cancer is one of the highest rate diseases in southeastern Asia. Recently, many of functional foods and alternative medicines are very popularly utilized to prevent chronic diseases and cancer in Taiwan. In this study, we wanted to select and develop some of novel effectual agents or phytochemicals of gamma-Mangostin for clinical management or prevent hepatocellular carcinoma cell (HCC).
METHODS AND RESULTS:
Lipid peroxidation (LPO) is an autocatalytic mechanism which induced tissue injure and carcinogenesis. In this study, the inhibitory activity of gamma-Mangostin on oxidative damage induced rat mitochondria LPO, the free radical scavenging of gamma-Mangostin and the apoptotic effects of gamma-Mangostin on HepG2 cells were investigated.
gamma-Mangostin processed activity to inhibit LPO and scavenge 2,2-diphenyl-1-picrylhydrazyl. gamma-Mangostin showed antiproliferative activity and induced nuclear condensation and apoptotic bodies appearance under Giemsa staining by microscopic observation. In addition, gamma-Mangostin showed increases of hypodiploid cells via propidium iodide, 2'7'-dichlorofluorescein diacetate, and 3,3'-dihexyloxacarbocyanine iodide staining by flow cytometry analysis in HepG2 cells.
CONCLUSIONS:
gamma-Mangostin has demonstrated free radical scavenging activity, and antiproliferative and apoptotic activity in HepG2 cells. The proof suggests that gamma-Mangostin is a lead compound candidate for clinical management or prevent HCC. | | J Med Assoc Thai. 2012 Dec;95 Suppl 12:S63-8. | | Mechanisms of vasorelaxation to gamma-mangostin in the rat aorta.[Pubmed: 23513467] | To investigate the effects of gamma-Mangostin on vascular tone and its mechanisms in the isolated rat aorta.
METHODS AND RESULTS:
Aortic rings from male Wistar rats were precontracted with methoxamine. Changes in tension were measured using an isometric force transducer and recorded on the MacLab recording system. Vasorelaxant effects of gamma-Mangostin were studied in the presence of 300 microM N(G)-nitro L-arginine methyl ester (L-NAME), 10 microM 1H-[1,2,4] oxadiazolo-[4,3-a] quinoxalin-1-one (ODQ), 10 microM indomethacin, 60 mM KCl, 5 mM tetraethylammonium (TEA), 10 microM glibenclamide, 1 mM4-aminopyridine (4-AP) or 30 microM barium chloride (BaCl2). Moreover the effects of gamma-Mangostin on contraction to CaCl2 were evaluated. gamma-Mangostin (1-100 microM) induced a concentration-dependent vasorelaxation in rat aortic rings precontracted with methoxamine. This effect was significantly reduced after removal of the endothelium and after pretreatment of the rings with L-NAME, ODQ, high KCl solution, or TEA. However, vasorelaxant responses to gamma-Mangostin were not altered by indomethacin, 4-AP, BaCl2 or glibenclamide. Moreover, contractions to CaCl2 (10 mM-30 mM) were reduced by pre-treatment with gamma-Mangostin (10 and 100 microM).
CONCLUSIONS:
gamma-Mangostin causes vasorelaxation which is mediated via the NO-cGMP pathway. Moreover activation of K+ channels and inhibition of extracellular Ca2+ influx from the extracellular space are largely involved in the relaxant effects of gamma-Mangostin. These data suggest that gamma-Mangostin may acts as an antihypertensive agent. | | Sci Rep . 2015 Aug 27;5:13570. | | Discovery of γ-Mangostin as an Amyloidogenesis Inhibitor[Pubmed: 26310724] | | Abstract
Transthyretin (TTR) is a homotetrameric protein involved in human hereditary amyloidoses. The discovery and development of small molecules that inhibit the amyloid fibril formation of TTR is one of the therapeutic strategies for these diseases. Herein, we discovered that γ-mangostin (γ-M) is an effective inhibitor against the amyloid fibril formation of V30M amyloidogenic TTR. In-vitro binding assays revealed that γ-M was the most potent of the selected xanthone derivatives, and it bound to the thyroxine (T4)-binding sites and stabilized the TTR tetramer. X-ray crystallographic analysis revealed the diagonal binding mode of γ-M and the two binding sites of chloride ions at the T4-binding site. One of the chloride ions was replaced with a water molecule in the α-mangostin complex, which is a methylated derivative of γ-M. The stronger inhibitory potency of γ-M could be explained by the additional hydrogen bonds with the chloride ion. The present study establishes γ-M as a novel inhibitor of TTR fibrillization. |
|
| In vivo: |
| Pharmacol Biochem Behav. 2010 Apr;95(2):166-72. | | New medicinal properties of mangostins: analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana L.[Pubmed: 20064550 ] | The fruit hull of Garcinia mangostana L. contains oxygenated and prenylated phenol derivatives, such as xanthones or xanthen-9H-ones, and is used by people in Southeast Asia as a traditional medicine for the treatment of abdominal pain, dysentery, wound infections, suppuration, and chronic ulcer. We isolated the active ingredients from the crude ethanol extract of G.mangostana L. (CEM) and investigated their analgesic effects and underlying mechanisms.
METHODS AND RESULTS:
CEM at intragastric (i.g.) doses of 0.5, 1, and 3 g/kg clearly exhibited antinociceptive effects in the hot-plate and acetic acid-induced writhing tests in mice. Two isolated compounds, alpha-mangostin and gamma-Mangostin, exhibited analgesic effects at doses of 25 and 50 mg/kg (i.g.) in the hot-plate and formalin tests, respectively. CEM at doses of 0.5, 1, and 3 g/kg significantly inhibited xylene-induced release of inflammatory mediators. CEM, alpha-mangostin, and gamma-Mangostin each dose-dependently demonstrated the ability to scavenge reactive oxygen species.
CONCLUSIONS:
In conclusion, our results demonstrate that CEM and mangostins possess potent peripheral and central antinociceptive effects in mice and suggest that xanthones may be developed as novel analgesics and anti-inflammatory drugs. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)