| Animal Research: |
| Inflamm Res. 1999 Apr;48(4):218-23. | | In vitro effect of the extract and the 1,7-dihydroxy-2,3-dimethoxy xanthone from Polygala cyparissias on the contractions induced by inflammatory mediators and ovalbumin in normal and actively sensitised trachea from guinea pig.[Pubmed: 10344473] |
METHODS AND RESULTS:
The hydroalcoholic extract of P. cyparissias (0.125 to 1 mg/ml), incubated with the guinea-pig trachea for 20 min, had no effect on the resting tone of the preparations, but caused a concentration-dependent, reversible and non competitive inhibition of contractions induced by acetylcholine, histamine, compound 48/80, bradykinin, substance P, prostaglandin E2 and the stable analogue of thromboxane A2 mimetic U 46619. The calculated mean IC50 values for the hydroalcoholic extract were: 0.37, 0.51, 0.06, 0.32, 0.48, 0.3 and 0.17 mg/ml, respectively. Also, the extract of P. cyparissias (0.125 to 0.5 mg/ml) antagonised, in a graded manner (IC50 of 0.46 mg/ml) ovalbumin-induced contractions in guinea-pig trachea obtained from animals which had been actively sensitised to this antigen. Pre-incubation of the preparations with the purifed xanthone isolated from P. cyparssias (2.5 to 80 microg/ml; 10.0 to 310.0 microM) caused significant and concentration-dependent, reversible and noncompetitive inhibition of the contractile responses elicited by acetylcholine, histamine, bradykinin, substance P, U 46619 and prostaglandin E2. The calculated mean IC50 values for these effects were: 132.0, 73.0, 9.2, 32.0, 110.6 and 66.0 microM, respectively. At very high concentrations (I55.0-620.0 microM) the xanthone also antagonised contraction induced by KCl in guinea-pig trachea (IC50 of 190.0 microM).
CONCLUSIONS:
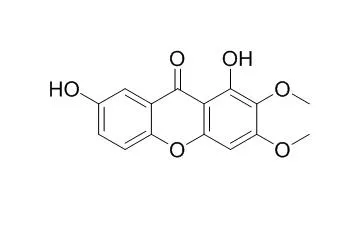
Taken together these and our previous in vivo results are consistent with the view that the active principles present in P. cyparissias, including the 1,7-Dihydroxy-2,3-dimethoxyxanthone, antagonise, in a non competitive but, reversible manner the contractions induced by chemical inflammatory mediators in the guinea pig trachea in vitro. Thus, these results might explain at least in part, the medicinal use of this plant in the management of inflammation, asthma and allergy. |
|
| Structure Identification: |
| Z Naturforsch C. 2004 May-Jun;59(5-6):335-8. | | Xanthones from Polygala alpestris (Rchb.).[Pubmed: 18998397] |
METHODS AND RESULTS:
Bioactivity-guided fractionation of Polygala alpestris L. (Rchb.) extracts led to the identification of two new xanthones, 1,3,7-trihydroxy-2,6-dimethoxyxanthone (1) and 2,3-methylenedioxy-4,7-dihydroxyxanthone (2). In addition five known compounds 3,4-dimethoxy-1,7-dihydroxyxanthone (3), 1,3-dihydroxy-7-methoxyxanthone (4), 1,7-Dihydroxy-2,3-dimethoxyxanthone (5), 3',6-O-disinapoyl sucrose (6) and 3',5'-dimethoxybiphenyl-4-olo (7) were isolated.
CONCLUSIONS:
The structures of the isolated compounds were established by means of high resolution mass spectrometry, mono- and bi-dimensional NMR spectroscopy.
All isolated compounds were tested for cytotoxic activity against three tumor cell lines (LoVo, HL-60, K 562). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)