| In vitro: |
| Planta Med. 2005 May;71(5):470-5. | | New indolopyridoquinazoline, benzo[c]phenanthridines and cytotoxic constituents from Zanthoxylum integrifoliolum.[Pubmed: 15931588] |
METHODS AND RESULTS:
Three new alkaloids, 7,8-dehydro-1-methoxyrutaecarpine, isodecarine, and 8-demethyloxychelerythrine, together with sixteen known compounds, norchelerythrine, oxychelerythrine, decarine, dihydrocherythrinylacetaldehyde, 6-acetonyldihydrochelerythrine, rutaecarpine, 1-Hydroxyrutaecarpine, gamma-fagarine, skimmianine, (-)-matairesinol, (-)-isoarctigenin, (+)-epipinoresinol, d-sesamin, lupeol, canthin-6-one, and arnottianamide have been isolated from the root bark of Zanthoxylum integrifoliolum. The structures of these new compounds were determined through spectral analyses.
CONCLUSIONS:
Among the isolates, 7,8-dehydro-1-methoxyrutaecarpine, norchelerythrine, oxychelerythrine, dihydrocherythrinylacetaldehyde, 6-acetonyldihydrochelerythrine, 1-Hydroxyrutaecarpine, gamma-fagarine, skimmianine, (-)-matairesinol, and canthin-6-one exhibited cytotoxicities (ED50 values < 4 microg/mL) against P-388 or HT-29 cell lines in vitro. | | Planta Med. 1996 Apr;62(2):175-6. | | Indolopyridoquinazoline alkaloids with antiplatelet aggregation activity from Zanthoxylum integrifoliolum.[Pubmed: 8657756] |
METHODS AND RESULTS:
Bioassay-guided fractionation led to the isolation of three indolopyridoquinazoline alkaloids, 1-Hydroxyrutaecarpine, rutaecarpine, and 1-methoxyrutaecarpine as the active principles of antiplatelet aggregation in vitro, from the chloroform-soluble part of the fruit of Zanthoxylum integrifoliolum (Rutaceae).
CONCLUSIONS:
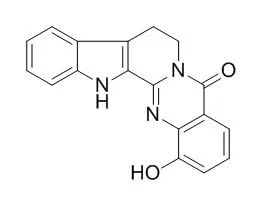
1-Hydroxyrutaecarpine exhibited antiplatelet activity induced by AA and showed an IC50 value of ca. 1-2 micrograms/ml. | | Planta Medica, 2012 , 78 (11) :1094-1094 | | The alkaloid 1-hydroxyrutaecarpine inhibits cathepsin activity[Reference: WebLink] |
Cathepsins B, L and K are cysteine proteases involved in various physiological and pathological processes. CatB and L are involved in tumoral processes while catK in cases of bone resorption.
METHODS AND RESULTS:
In a search for cathepsin inhibitors we have isolated from Metrodorea stipullaris, a number of compounds, among them the alkaloid 1-Hydroxyrutaecarpine which displayed moderate inhibitory activity on those enzymes. The alkaloid showed inhibition of 79% on cathepsin B, 100% on cathepsin L and 87.5% on cathepsin K, at the concentration of 125μg/ml for the three cathepsins. IC50 experiments were run and the values obtained are described in the table below. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)