| Structure Identification: |
| J Org Chem. 2000 Jun 16;65(12):3804-10. | | Alkaloid-fullerene systems through photocycloaddition reactions.[Pubmed: 10864768] | The photocycloaddition of tertiary amines to ¿60fullerene (C(60)) is an interesting and useful reaction.
METHODS AND RESULTS:
We wished to extend the applications of this type of reaction through an investigation of the photoaddition of alkaloids to C(60) for the purpose of synthesizing novel and complex photoadducts that are difficult to obtain by usual methods. Irradiation of tazettine (2) or gramine (3) with C(60) in toluene leads to formation of one monoadduct (6 or 7), whereas scandine (1a) or 10-Hydroxyscandine (1b) reacts with C(60) photochemically to give two products, the expected ¿6,6 monoadduct (5a, 5b) and a new type of monoadduct with a bis-¿6, 6 closed structure (4a, 4b).These new structures were characterized by UV-vis, FT-IR, (1)H NMR, (13)C NMR, (1)H-(1)H COSY, ROESY, HMQC (heteronuclear multiple-quantum coherence), and HMBC (heteronuclear multiple-bond connectivity) spectroscopy.
The techniques of time-of-flight secondary ion MS (TOF-SIMS) and field desorption MS (FD-MS) were used for the mass determination.
CONCLUSIONS:
(3)He NMR analysis of the product mixture from photoaddition of 1a to C(60) containing a (3)He atom ((3)He@C(60)) led to two peaks at -9.091 and -11.090 ppm relative to gaseous (3)He, consistent with formation of a ¿6, 6-closed monoadduct and a bis-¿6,6 closed adduct. Presumably, the bis-¿6, 6 closed adducts are formed by an intramolecular ¿2 + 2 cycloaddition of the vinyl group to the adjacent 6,6-ring junction of C(60) after the initial photocycloaddition. | | Planta Med. 1988 Aug;54(4):315-7. | | Study on the Alkaloids of Melodinus tenuicaudatus.[Pubmed: 17265274 ] |
METHODS AND RESULTS:
Fourteen alkaloids were isolated from the stem bark of MELODINUS TENUICAUDATUS Tsiang et P. T. Li. Eleven of them were identified as known alkaloids, namely, scandine ( 2), Delta (14)-eburnamine ( 4), vindolinine N(b)-oxide ( 5), 11-methoxytabersonine ( 6), vindolinine ( 7), EPI-vindolinine N(b)-oxide ( 8), hazuntine ( 9), compactinervine ( 10), 11-hydroxytabersonine ( 11), Delta (14)-vincine ( 12), and normacusine B ( 14).
CONCLUSIONS:
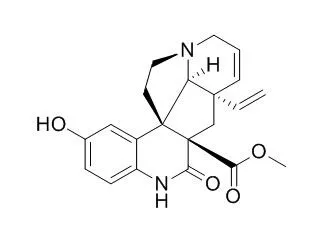
Two alkaloids were new: 10-Hydroxyscandine ( 1), and the dimer, tenuicausine ( 3); their structures were elucidated by spectroscopic and chemical methods. One alkaloid ( 13) occurring in trace amounts, could not be identified. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)