| Structure Identification: |
| The Journal of Organic Chemistry, 1968, 33(10):3735-3738. | | The isolation and structural elucidation of novel derivatives of aristolochic acid from Aristolochia indica.[Reference: WebLink] | The isolation and structural elucidation are reported of three new companion Aristolochic acid Derivatives, aristolochic acid D (4), aristolochic acid-D methyl ether lactam (6), and aristololactam β-D-glucoside (8).
METHODS AND RESULTS:
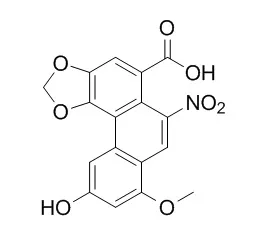
Aristolochic acid D was assigned the molecular formula C17H11NO8 on the basis of elemental analysis and nmr spectroscopy. Methylation with diazomethane yielded the dimethyl derivative 5, and hydrogenation of 5 yielded aristolochic acid-D methyl ether lactam (6), identical with the material of natural origin. The structure of 5 was initially deduced on the basis of spectral evidence, and confirmed by direct comparison with a sample prepared by total synthesis. Spectral arguments are presented which favor structure 4 for Aristolochic acid D.
CONCLUSIONS:
Aristololactam β-D-glucoside (8) was characterized by elemental and nmr, ir, and uv spectral analysis. Acetylation gave the tetraacetate, 9. Lithium aluminum hydride reduction of 8, followed by mild acid hydrolysis, gave α-D-glucose and the aglycone 11, characterized by comparison with the product obtained by lithium aluminum hydride reduction of aristololactam. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)