| Structure Identification: |
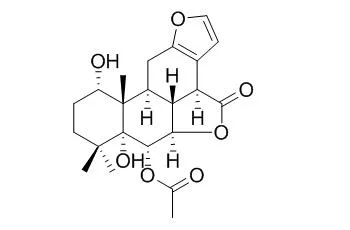
| J Nat Prod. 2013 Dec 27;76(12):2210-8. | | Caesalminaxins A-L, cassane diterpenoids from the seeds of Caesalpinia minax.[Pubmed: 24303808 ] | Fourteen new cassane diterpenoids, caesalminaxins A-L (1-14), and three known compounds were isolated from the seeds of Caesalpinia minax.
METHODS AND RESULTS:
Among the new diterpenoids, compounds 3 and 4 possess a rare spiro C/D ring system. The C-16 epimeric mixture 1/2 has an unprecedented carbon skeleton, presumably derived from 3 by cleavage of the C-13-C-14 bond. Compound 5 is the first example of a cassane diterpenoid with a spiro A/B ring system. The structures of the compounds were elucidated on the basis of 1D and 2D NMR analysis, and the absolute configurations of 3, 4, 9, and 11 were determined by single-crystal X-ray crystallography. Biosynthesis pathways for 1/2, 3, and 5 are postulated.
CONCLUSIONS:
Compounds 4, 8, and the known Bonducellpin D exhibited moderate activity against four tested human cancer cell lines, HepG-2, K562, HeLa, and Du145. | | Bioorg Med Chem. 2002 Jul;10(7):2161-70. | | Molecular structures and antiviral activities of naturally occurring and modified cassane furanoditerpenoids and friedelane triterpenoids from Caesalpinia minax.[Pubmed: 11983512] |
METHODS AND RESULTS:
Further investigation of the active components of the chloroform fraction of the seeds of Caesalpinia minax led to the isolation of a new cassane furanoditerpenoid, caesalmin H (1), together with two known furanoditerpenoid lactones, caesalmin B (2) and Bonducellpin D (3). Reduction of the naturally abundant caesalmin D (9), E (10) and F (11) resulted in three new furanoditerpenoid derivatives 4-6. Phytochemical study of the stem of the same plant and subsequent reduction afforded two friedelane triterpenoids (7-8), which were identified by spectroscopic methods. Compounds 1-2 and 4-8 were corroborated by single crystal X-ray analysis.
CONCLUSIONS:
The factors governing the reduction of cassane furanoditerpenoids and friedelane triterpenoids were investigated by correlating the crystallographic results with density functional theory. The inhibitory activities of 2-8 on the Para3 virus were evaluated by cytopathogenic effects (CPE) reduction assay. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)