| Kinase Assay: |
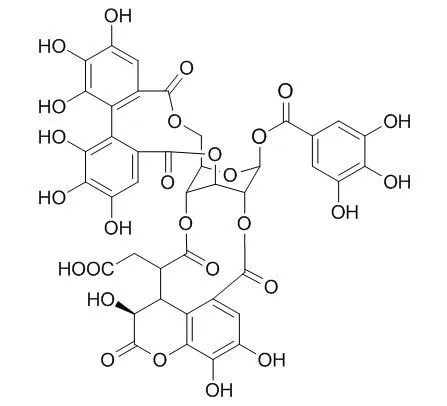
| Biomol Ther (Seoul). 2014 Jul;22(4):275-81. | | Neuroprotective Effect of Chebulagic Acid via Autophagy Induction in SH-SY5Y Cells.[Pubmed: 25143804] | Autophagy is a series of catabolic process mediating the bulk degradation of intracellular proteins and organelles through formation of a double-membrane vesicle, known as an autophagosome, and fusing with lysosome. Autophagy plays an important role of death-survival decisions in neuronal cells, which may influence to several neurodegenerative disorders including Parkinson's disease. Chebulagic acid, the major constituent of Terminalia chebula and Phyllanthus emblica, is a benzopyran tannin compound with various kinds of beneficial effects. This study was performed to investigate the autophagy enhancing effect of Chebulagic acid on human neuroblastoma SH-SY5Y cell lines.
METHODS AND RESULTS:
We determined the effect of Chebulagic acid on expression levels of autophago-some marker proteins such as, DOR/TP53INP2, Golgi-associated ATPase Enhancer of 16 kDa (GATE 16) and Light chain 3 II (LC3 II), as well as those of its upstream pathway proteins, AMP-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR) and Beclin-1. All of those proteins were modulated by Chebulagic acid treatment in a way of enhancing the autophagy. Additionally in our study, Chebulagic acid also showed a protective effect against 1-methyl-4-phenylpyridinium (MPP(+)) - induced cytotoxicity which mimics the pathological symptom of Parkinson's disease. This effect seems partially mediated by enhanced autophagy which increased the degradation of aggregated or misfolded proteins from cells.
CONCLUSIONS:
This study suggests that Chebulagic acid is an attractive candidate as an autophagy-enhancing agent and therefore, it may provide a promising strategy to prevent or cure the diseases caused by accumulation of abnormal proteins including Parkinson's disease. | | Biofactors. 2014 Nov-Dec;40(6):646-57. | | Chebulagic acid from Terminalia chebula enhances insulin mediated glucose uptake in 3T3-L1 adipocytes via PPARγ signaling pathway.[Pubmed: 25529897] | The thiazolidinedione (TZDs) class of drugs are very effective for the treatment of type 2 diabetes mellitus (T2DM). But due to the adverse effects of synthetic TZDs, their use is strictly regulated. The therapeutic actions of TZDs are mediated via modulation of peroxisome proliferator-activated receptor gamma (PPARγ). Naturally occurring PPARγ modulators are more desirable as they lack the serious adverse effects caused by TZDs. This has prompted the exploitation of medicinal plants used in traditional medicine, for their potential PPARγ activity.
METHODS AND RESULTS:
In the present work, we studied Chebulagic acid (CHA) isolated from fruits of Terminalia chebula with respect to its effect on adipogenesis, glucose transport, and endocrine function of adipocyte. The mRNA expression profile of PPARγ target gene CCAAT/enhancer-binding protein alpha (C/EBP-α) was analyzed by qRT-PCR. The putative binding mode and the potential ligand-target interactions of CHA, with PPARγ was analyzed using docking software (Autodock and iGEMDOCKv2). The results showed that CHA enhances PPARγ signaling and adipogenesis dose dependently but in a moderate way, less than rosiglitazone. GLUT4 expression and adiponectin secretion was increased by CHA treatment. The mRNA expression of PPARγ target gene C/EBP-α was increased in CHA -treated adipocytes.
CONCLUSIONS:
The comparison of results of various parameters of adipogenesis, insulin sensitivity, endocrine function and molecular docking experiments of roziglitazone and Chebulagic acid indicate that the latter behaves like partial PPARγ agonist which could be exploited for phytoceutical development against T2DM. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)