| Kinase Assay: |
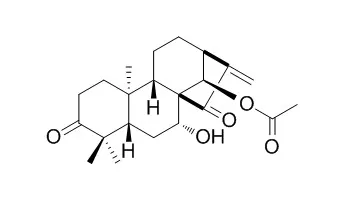
| Cell Physiol Biochem. 2017;44(6):2091-2104. | | Glaucocalyxin B Alleviates Lipopolysaccharide-Induced Parkinson's Disease by Inhibiting TLR/NF-κB and Activating Nrf2/HO-1 Pathway.[Pubmed: 29241205] | Parkinson's disease (PD) is a common neurodegenerative disease in the old population, characterized by dopaminergic neuron loss, inflammation and oxidative stress injury in the substantia nigra. Glaucocalyxin B (GLB), an ent-kauranoid diterpenoid isolated from Rabdosia japonica, has anti-inflammation and anti-tumor effects. However, its effects on PD remain unclear.
METHODS AND RESULTS:
PD was introduced in rats via injection of lipopolysaccharide (LPS) into cerebral corpus striatum, and GLB was given intracerebroventricularly to these rats. Their walking, climbing and sensory states were detected by Stepping, Whisker and Cylinder Tests. The expression of tyrosine hydroxylase (TH), glial fibrillary acidic protein (GFAP), CD11b and ionized calcium binding adaptor molecule (IBA)-1 were detected by immunohischemical staining. The levels of a series of inflammatory factors, oxidative stress-related factors and apoptosis-related factors were measured by real-time PCR, immunoblotting and ELISA. In addition, Toll-like receptor (TLR)/nuclear factor kappa B (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase (HO)-1 pathways were investigated to illustrate the underlying mechanism. In vitro, microglial cells exposed to LPS were treated with GLB. The injection of LPS caused walking, climbing and sensory disturbances in rats, induced inflammation, oxidative stress response and apoptosis, and activated TLR/NF-κB and Nrf2/ HO-1 pathways in the cerebral tissue. GLB administration attenuated LPS-induced alterations. The TLR/NF-κB pathway was deactivated and Nrf2/HO-1 was activated after application of GLB. In vitro, cytotoxic effects induced by the conditioned medium derived from microglial cells exposed to LPS in PC12 cells were attenuated by GLB.
CONCLUSIONS:
GLB suppresses LPS-induced PD symptoms by modification of TLR/NF-κB and Nrf2/HO-1 pathways in vivo and in vitro. | | Pharmacol Res. 2018 Mar;129:262-273. | | Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer's disease.[Pubmed: 29179999 ] | Alzheimer's disease (AD) is the most prevalent form of dementia. The exact pathophysiology of this disease remains incompletely understood and safe and effective therapies are required. AD is highly correlated with neuroinflammation and oxidative stress in brain causing neuronal loss.
METHODS AND RESULTS:
Nuclear factor of activated B-cells (NF-κB) is involved in physiological inflammatory processes and thus representing a promising target for inflammation-based AD therapy. Phytochemicals are able to interfere with the NF-κB pathway.
They inhibit the phosphorylation or the ubiquitination of signaling molecules, and thus, inhibit the degradation of IκB. The translocation of NF-κB to the nucleus and subsequent transcription of pro-inflammatory cytokines are inhibited by the actions of phytochemicals. Additionally, natural compounds preventing the interaction of NF-κB can block NF-κB's transcriptional activity by inhibiting its binding to target DNA. Many polyphenols including curcumin, resveratrol, pterostilbene, punicalagin, macranthoin G, salidroside, 4-O-methylhonokiol, lycopene, genistein, obovatol and gallic acid were reported as potent NF-κB inhibitors for AD treatment. Several alkaloids such as galantamine, Glaucocalyxin B, tetrandrine, berberine, oridonin, anatabine have been shown anti-inflammatory effects in AD models in vitro as well as in vivo. Besides, vitamins, tanshinone IIA, artemisinin, dihydroasparagusic acid, geniposide, xanthoceraside, l-theranine, 1,8-cineole and paeoniflorin were described as promising NF-κB inhibitors.
CONCLUSIONS:
In conclusion, natural products from plants represent interesting candidates for AD treatment. They may qualify as promising compounds for the development of derivatives providing enhanced pharmacological features. | | Mol Med Rep. 2016 Aug;14(2):1751-5. | | Glaucocalyxin B induces apoptosis and autophagy in human cervical cancer cells.[Pubmed: 27356884] | Glaucocalyxin (Gln), an ent‑kaurane diterpenoid isolated from the Chinese traditional medicine, Rabdosia japonica, represents a novel class of anticancer drugs. Glaucocalyxin A(GlnA) is one of the three major forms of Gln and has demonstrated potent anticancer effects in a variety of cancer types. Glaucocalyxin B(GlnB) has only one structural difference from GlnA, an acetylated hydroxyl group at C14. This acetyl group results in high liposolubility and may enhance the antitumor activity of ent‑kaurane diterpenoid GlnB. However, few studies have reported the role of GlnB in cancer.
METHODS AND RESULTS:
The present study investigated the effect of GlnB in cervical cancer proliferation and cell death. Treatment with GlnB inhibits the proliferation of HeLa and SiHa cervical cancer cell lines in a dose‑dependent manner, as assessed by 3‑(4,5‑dimethylthiazol-2‑yl)-2,5 diphenyl tetrazolium bromide assays. In addition, GlnB increases the apoptotic cell population of HeLa and SiHa cells, as determined by fluorescence‑activated cell sorting analysis and enhanced poly (ADP‑ribose) polymerase 1 cleavage by western blotting. GlnB also induces increased light chain 3 II/I protein cleavage in both cells, indicating the induction of autophagy. Furthermore, GlnB treatment increased the expression of phosphatase and tensin homolog and decreased the expression of phosphorylated‑protein kinase B (Akt) in HeLa and SiHa cells, as assessed by western blotting.
CONCLUSIONS:
Taken together, the present results demonstrated that GlnB inhibited the proliferation of human cervical cancer cells in vitro through the induction of apoptosis and autophagy, which may be mediated by the phosphatidylinositol‑4,5‑bisphosphate 3‑kinase/Akt signaling pathway. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)