| In vitro: |
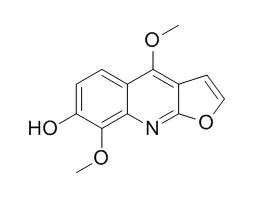
| Planta Med. 2004 Jun;70(6):531-5. | | Photo-activated DNA binding and antimicrobial activities of furoquinoline and pyranoquinolone alkaloids from rutaceae.[Pubmed: 15229804] | To find novel photo-active compounds of potential use in photochemotherapy from higher plants, photo-activated antimicrobial and DNA binding activities of the furoquinolines, skimmianine, kokusaginine, and Haplopine, and a pyranoquinolone, flindersine, from two species of Rutaceae plants were investigated.
METHODS AND RESULTS:
TLC overlay assays against a methichillin-resistant strain of Staphylococcus aureus and Candida albicans were employed to test antimicrobial properties. All of the tested compounds showed photo-activated antimicrobial activity against S. aureus in the order of kokusaginine > Haplopine, flindersine > skimmianine. Weaker activity was found for C. albicans. Photo-activated DNA binding activity of these compounds was investigated by a method using restriction enzymes and a specially designed 1.5 kb DNA fragment. Kokusaginine showed inhibition against all of the 16 restriction enzymes. Haplopine showed a similar inhibition pattern but the binding activity against Asc I and Sma I with restriction sequences consisting only of G and C was very weak. Skimmianine showed binding activity against Xba I, BciV I, Sal I, Pst I, Sph I and Hind III, but very weak or no activity was found for the other restriction enzymes. A pyranoquinolone, flindersine, showed no activity against any of the restriction enzymes.
CONCLUSIONS:
Photo-activated DNA binding activity of furoquinolines was therefore in the order of kokusaginine > Haplopine > skimmianine, which was the same order as their photo-activated antimicrobial activity against S. aureus. | | Chem Biodivers. 2017 Jul;14(7). | | Melanogenesis-Inhibitory and Cytotoxic Activities of Limonoids, Alkaloids, and Phenolic Compounds from Phellodendron amurense Bark.[Pubmed: 28425165 ] | Four limonoids, 1 - 4, five alkaloids, 5 - 9, and four phenolic compounds, 10 - 13, were isolated from a MeOH extract of the bark of Phellodendron amurense (Rutaceae).
METHODS AND RESULTS:
Among these, compound 13 was new, and its structure was established as rel-(1R,2R,3R)-5-hydroxy-3-(4-hydroxy-3-methoxyphenyl)-6-methoxy-1-(methoxycarbonylmethyl)indane-2-carboxylic acid methyl ester (γ-di(methyl ferulate)) based on the spectrometric analysis. Upon evaluation of compounds 1 - 13 against the melanogenesis in the B16 melanoma cells induced with α-melanocyte-stimulating hormone (α-MSH), four compounds, limonin (1), noroxyhydrastinine (6), Haplopine (7), and 4-methoxy-1-methylquinolin-2(1H)-one (8), exhibited potent melanogenesis-inhibitory activities with almost no toxicity to the cells. Western blot analysis revealed that compound 6 inhibited melanogenesis, at least in part, by inhibiting the expression of protein levels of tyrosinase, TRP-1, and TRP-2 in α-MSH-stimulated B16 melanoma cells. In addition, when compounds 1 - 13 were evaluated for their cytotoxic activities against leukemia (HL60), lung (A549), duodenum (AZ521), and breast (SK-BR-3) cancer cell lines, five compounds, berberine (5), 8, canthin-6-one (9), α-di-(methyl ferulate) (12), and 13, exhibited cytotoxicities against one or more cancer cell lines with IC50 values in the range of 2.6 - 90.0 μm.
CONCLUSIONS:
In particular, compound 5 exhibited strong cytotoxicity against AZ521 (IC50 2.6 μm) which was superior to that of the reference cisplatin (IC50 9.5 μm). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)