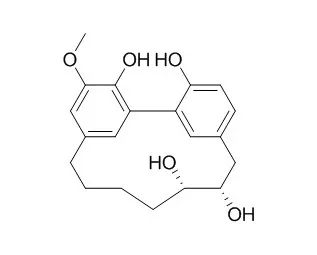
Bioassay-guided fractionation of the roots of Myrica adenophora led to isolation of 24 known compounds and hitherto unknown compounds, including three A-type proanthocyanidins [adenodimerins A-C], two esters of sucrose [myricadenins A and B ], and the phenolic glycoside 6'-O-galloyl orbicularin.
METHODS AND RESULTS:
Spectroscopic analyses were used to determine their structures. Adenodimerin A, myricananin C, and myricetin showed strong 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities, with SC50 values of 7.9, 16.3, and 15.9 μM, respectively. Adenodimerin A, myricanone, myricananin C, (-)-myricanol, myricanol 11-O-β-D-glucopyranoside, and myricetin showed stronger 2,2'-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging activities than the positive control, with SC50 values of 7.5, 19.6, 12.0, 22.3, 19.6, and 15.6 μM, respectively. 5-Deoxymyricanone, porson, 12-hydroxymyricanone (-)-myricanol, and (+)-galeon exhibited anti-tubercular activity against Mycobacterium tuberculosis H37Rv in vitro and MICs values of 25.8, 40.0, 35.8, 30.0, and 15.0 μg/mL, respectively. Myricananin A , myricanone, myricananin C, and (-)-myricanol exhibited anti-inflammatory activities in the iNOS assay with EC50 values of 18.1, 1.00, 13.0, and 7.5 μM, respectively. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)