| In vitro: |
| Molecules . 2020 Nov 23;25(22):5472. | | Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H 2 O 2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway[Pubmed: 33238414] | | Oleuropein, a glycosylated secoiridoid present in olive leaves, is known to be an important antioxidant phenolic compound. We studied the antioxidant effect of low doses of Oleuropein aglycone (3,4-DHPEA-EA) and Oleuropein aglycone peracetylated (3,4-DHPEA-EA(P)) in murine C2C12 myocytes treated with hydrogen peroxide (H2O2). Both compounds were used at a concentration of 10 μM and were able to inhibit cell death induced by the H2O2 treatment, with 3,4-DHPEA-EA(P) being more. Under our experimental conditions, H2O2 efficiently induced the phosphorylated-active form of JNK and of its downstream target c-Jun. We demonstrated, by Western blot analysis, that 3,4-DHPEA-EA(P) was efficient in inhibiting the phospho-active form of JNK. This data suggests that the growth arrest and cell death of C2C12 proceeds via the JNK/c-Jun pathway. Moreover, we demonstrated that 3,4-DHPEA-EA(P) affects the myogenesis of C2C12 cells; because MyoD mRNA levels and the differentiation process are restored with 3,4-DHPEA-EA(P) after treatment. Overall, the results indicate that 3,4-DHPEA-EA(P) prevents ROS-mediated degenerative process by functioning as an efficient antioxidant. | | Food Chem Toxicol . 2019 Jul;129:1-12. | | Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ 1-42 aggregation[Pubmed: 30995514] | | Oleuropein aglycone (OleA), the most abundant polyphenol in extra virgin olive oil (EVOO), and Hydroxythyrosol (HT), the OleA main metabolite, have attracted our interest due to their multitarget effects, including the interference with amyloid aggregation path. However, the mechanistic details of their anti-amyloid effect are not known yet. We report here a broad biophysical approach and cell biology techniques that enabled us to characterize the different molecular mechanisms by which OleA and HT modulate the Aβ1-42 fibrillation, a main histopathological feature of Alzheimer's disease (AD). In particular, OleA prevents the growth of toxic Aβ1-42 oligomers and blocks their successive growth into mature fibrils following its interaction with the peptide N-terminus, while HT speeds up harmless fibril formation. Our data demonstrate that, by stabilizing oligomers and fibrils, both polyphenols reduce their seeding activity and aggregate/membrane interaction on human neuroblastoma SH-SY5Y cells. These findings highlight the great potential of EVOO polyphenols and offer the possibility to validate and to optimize their use for possible AD prevention and therapy. | | Food Chem Toxicol . 2019 Jul;129:1-12. | | Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ 1-42 aggregation[Pubmed: 30995514] | | Oleuropein aglycone (OleA), the most abundant polyphenol in extra virgin olive oil (EVOO), and Hydroxythyrosol (HT), the OleA main metabolite, have attracted our interest due to their multitarget effects, including the interference with amyloid aggregation path. However, the mechanistic details of their anti-amyloid effect are not known yet. We report here a broad biophysical approach and cell biology techniques that enabled us to characterize the different molecular mechanisms by which OleA and HT modulate the Aβ1-42 fibrillation, a main histopathological feature of Alzheimer's disease (AD). In particular, OleA prevents the growth of toxic Aβ1-42 oligomers and blocks their successive growth into mature fibrils following its interaction with the peptide N-terminus, while HT speeds up harmless fibril formation. Our data demonstrate that, by stabilizing oligomers and fibrils, both polyphenols reduce their seeding activity and aggregate/membrane interaction on human neuroblastoma SH-SY5Y cells. These findings highlight the great potential of EVOO polyphenols and offer the possibility to validate and to optimize their use for possible AD prevention and therapy. | | J Biomol Struct Dyn . 2021 Mar;39(4):1259-1270. | | Computational investigation on the effect of Oleuropein aglycone on the α-synuclein aggregation[Pubmed: 32041489] | | Parkinson's disease (PD) is considered to be the second most common progressive neurodegenerative brain disorder after Alzheimer's disease, which is caused by misfolding and aggregation of Alpha-synuclein (α-synuclein). It is characterized by distinct aggregated fibrillary form of α-synuclein known as the Lewy bodies and Lewy neurites. The most promising approach to combat PD is to prevent the misfolding and subsequent aggregation of α-synuclein. Recently, Oleuropein aglycone (OleA) has been reported to stabilize the monomeric structure of α-synuclein, subsequently favoring the growth of nontoxic aggregates. Therefore, understanding the conformational dynamics of α-synuclein monomer in the presence of OleA is significant. Here, we have investigated the effect of OleA on the conformational dynamics and the aggregation propensity of α-synuclein using molecular dynamics simulation. From molecular dynamics trajectory analysis, we noticed that when OleA is bound to α-synuclein, the intramolecular distance between non-amyloid-β component domain and C-terminal domain of α-synuclein was increased, whereas long-range hydrophobic interactions between the two region were reduced. Oleuropein aglycone was found to interact with the N-terminal domain of α-synuclein, making this region unavailable for interaction with membranes and lipids for the formation of cellular toxic aggregates. From the binding-free energy analysis, we found binding affinity between α-synuclein and OleA to be indeed high (ΔGbind = -12.56 kcal mol-1 from MM-PBSA and ΔGbind = -27.41 kcal mol-1from MM-GBSA). Our findings in this study thus substantiate the effect of OleA on the structure and stabilization of α-synuclein monomer that subsequently favors the growth of stable and nontoxic aggregates.Communicated by Ramaswamy H. Sarma. | | Food Funct . 2017 Dec 13;8(12):4684-4692. | | Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives[Pubmed: 29160876] | | Oleuropein is the most important phenolic compound present in olive cultivars, but it is scarcely present in extra virgin olive oil (EVOO) due to its high hydrophilicity and degradability. Thus, a set of Oleuropein aglycone derivatives were synthesized by transacetylation under mild conditions with the aim of circumventing these drawbacks and making the active moiety in oleuropein suitable to be added to food fats. The Oleuropein aglycone (closed ring form) is obtained by hydrolyzing oleuropein using Lewis acid catalysis. Then, the permeation profiles as well as the antioxidant capacity of the Oleuropein aglycone derivatives were evaluated by ORAC, DPPH assays and by ROS formation using the SH-SY5Y cell line. The biological activities of the obtained compounds exhibited a dependence on their level of lipophilicity. |
|
| In vivo: |
| J Nutr Biochem . 2017 Feb;40:209-218. | | Oleuropein aglycone enhances UCP1 expression in brown adipose tissue in high-fat-diet-induced obese rats by activating β-adrenergic signaling[Pubmed: 27951473] | | Oleuropein is the pungent principle of raw olives. Oleuropein aglycone (OA) is a major phenolic compound in extra virgin olive oil and the absorbed form of oleuropein. We aimed to determine the mechanism underlying the nutritional effects of oleuropein and OA on interscapular brown adipose tissue (IBAT) in rats with high-fat (HF) diet-induced obesity by examining the agonistic activity of oleuropein and OA toward the transient receptor potential ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1). Four-week-old male Sprague-Dawley rats were fed an HF (palm oil 30% wt:wt) diet alone or with oleuropein (HF-O, 1 g/kg diet) for 28 days. In rats fed HF-O compared to HF, urinary noradrenaline, adrenaline and UCP1 levels in IBAT were significantly higher, whereas plasma leptin levels and the total weight of the abdominal cavity adipose tissue were significantly lower. In anaesthetized 7-week-old male Sprague-Dawley rats, the OA (3.8 mg of intravenous injection)-induced increase in plasma noradrenaline secretion was suppressed by TRPA1 or TRPV1 antagonist and by a β2- or β3-adrenoceptor antagonist. Furthermore, OA-activated rat and human TRPV1s expressed on HEK293 cells at the same level as zingerone (pungent component in ginger). OA also activated humanTRPA1, and its potency was approximately 10-fold stronger than that for TRPV1. These findings suggest that OA is the agonist of both TRPA1 and TRPV1 and that OA enhances UCP1 expression in IBAT with a concomitant decrease in the visceral fat mass of HF-diet-induced obese rats through enhanced noradrenaline secretion via β-adrenergic action following TRPA1 and TRPV1 activation. |
|


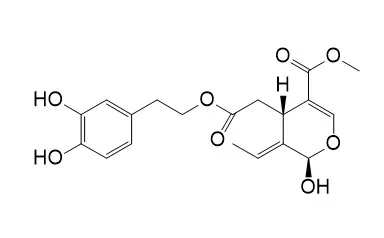



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)