| Structure Identification: |
| Chem Biodivers. 2007 Feb;4(2):145-53. | | DNA-binding affinities and sequence specificities of protoberberine alkaloids and their demethylated derivatives: a comparative study.[Pubmed: 17311227] |
METHODS AND RESULTS:
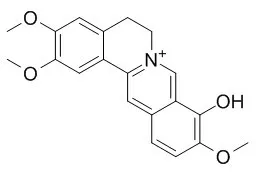
Berberrubine (1a), jatrorubine (2a), and Palmatrubine (3a) have been chemically prepared by partial demethylation of berberine (1), jatrorrhizine (2), and palmatine (3), respectively. Their interactions with calf thymus (CT) DNA, poly(dA-dT)poly(dA-dT), poly(dG-dC)poly(dG-dC), and eight AT-rich 12-mer double-stranded DNAs have been investigated by means of competitive ethidium bromide (EB) displacement experiments. The results showed that DNA-binding affinities of these protoberberine alkaloids have been significantly improved by partial demethylation, and that all of these alkaloids have the preferable binding affinities with AT-rich DNA. Especially, the sequence specificities of DNA-binding of demethylated derivatives 1a, 2a, and Palmatrubine had changed to a certain extent when compared with the parent alkaloids 1, 2, and 3, respectively. The binding mode of these alkaloids was further confirmed by UV spectroscopic titration experiments.
CONCLUSIONS:
All the compounds bind to double-stranded DNA most probably via an intercalating mode. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)