| Description: |
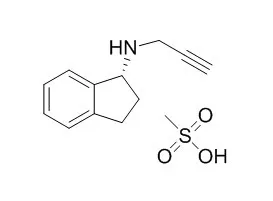
Rasagiline mesylate is a potent, selective, non-reversible MAO-B inhibitor, with neuroprotective activities, with anti-Parkinson activity. Rasagiline mesylate exhibits neuroprotective and anti-apoptotic activity against several neurotoxins in cell culture. |
| Targets: |
MAO | ROS |
| In vitro: |
| Int J Pharm Investig. 2015 Apr-Jun;5(2):87-91. | | Effect of the moist-heat sterilization on fabricated nanoscale solid lipid particles containing rasagiline mesylate.[Pubmed: 25838993 ] | Nanoscale solid lipid particles of Rasagiline mesylate (RM) were fabricated by microemulsion technique. The nanoscale particle must be sterile for intravenous administration, and several approaches are available for sterilization. However, the selection of sterilization technique for the fabricated RM loaded nanoscale solid lipid particles mainly depends on the nature of the drug that needs to be encapsulated and release pattern of the polymer.
METHODS AND RESULTS:
We have preferred moist heat sterilization, as it is the most convenient and the composition of the carrier and incorporated drug should remain unchanged and the incorporated drug should not leak out of the drug carrier. The physical and chemical stability of RM loaded nanoscale solid lipid particles investigated during sterilization and to determine the average mean particle size, polydispersity index, zeta potential (ZP), transmission electron microscopy (TEM), entrapment efficiency (EE), and drug content after autoclaving.
There were no significant changes in the average mean particle size, polydispersity index, ZP, TEM, EE, and drug content of RM loaded nanoscale solid lipid particles after autoclaving (121°C for 20 min [15 lbs]).
CONCLUSIONS:
These observations suggest that the moist heat sterilization by autoclaving is the most suitable method for nanoscale solid lipid formulations. | | Int J Pharm. 2012 Nov 15;438(1-2):266-78. | | Controlled release of rasagiline mesylate promotes neuroprotection in a rotenone-induced advanced model of Parkinson's disease.[Pubmed: 22985602] | Microencapsulation of Rasagiline mesylate (RM) into PLGA microspheres was performed by method A (O/W emulsion) and method B (W/O/W double emulsion). The best formulation regarding process yield, encapsulation efficiency and in vitro drug release was that prepared with method A, which exhibited constant drug release for two weeks (K(0)=62.3 μg/day/20mg microspheres).
METHODS AND RESULTS:
Exposure of SKN-AS cells to peroxide-induced oxidative stress (1 mM) resulted in cell apoptosis which was significantly reduced by RM (40.7-102.5 μM) as determined by cell viability, ROS production and DNA fragmentation. Daily doses of rotenone (2 mg/kg) given i.p. to rats for 45 days induced neuronal and behavioral changes similar to those occurring in PD. Once an advanced stage of PD was achieved, animals received RM in saline (1 mg/kg/day) or encapsulated within PLGA microspheres (amount of microspheres equivalent to 15 mg/kg RM given on days 15 and 30). After 45 days RM showed a robust effect on all analytical outcomes evaluated with non-statistically significant differences found between its administration in solution or within microparticles however; with this controlled release system administration of RM could be performed every two weeks thereby making this new therapeutic system an interesting approach for the treatment of PD. |
|
| In vivo: |
| Clin Neuropharmacol. 2000 Nov-Dec;23(6):324-30. | | Rasagiline mesylate, a new MAO-B inhibitor for the treatment of Parkinson's disease: a double-blind study as adjunctive therapy to levodopa.[Pubmed: 11575866] | Rasagiline mesylate (TVP-1012) is a potent, selective, non-reversible MAO-B inhibitor, without the tyramine-potentiating effect and with neuroprotective activities. The benefit of rasagiline as monotherapy in patients with early Parkinson's disease (PD) has already been reported.
METHODS AND RESULTS:
To evaluate the safety, tolerability, and clinical effect of rasagiline as adjunctive therapy to levodopa, a multicenter, double-blind, randomized, placebo-controlled, parallel-group study (0.5, 1, and 2 mg/d) was conducted for 12 weeks in 70 patients with PD (mean age, 57.4 y; mean disease duration, 5.7 y; 32 patients had motor fluctuations). A beneficial clinical effect was observed in fluctuating patients treated with rasagiline (all doses), expressed as a decrease in total Unified Parkinson's Disease Rating Scale (UPDRS) score (23.0% vs 8.5% in the placebo group). The treatment effect was still evident 6 weeks after drug discontinuation (in all doses). The safety and tolerability of rasagiline were good. Adverse events were no different than those of patients taking placebo. Almost complete platelet MAO-B inhibition was obtained at all rasagiline doses.
CONCLUSIONS:
This study has demonstrated that rasagiline (up to 2 mg/day) has a good safety profile and a beneficial clinical effect in fluctuating patients with PD when given as an add-on to chronic levodopa therapy. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)