| In vitro: |
| Phytochemistry. 2014 Dec;108:137-46. | | Diversity of pyrrolizidine alkaloids in native and invasive Senecio pterophorus (Asteraceae): implications for toxicity.[Pubmed: 25269662] | Changes in plant chemical defenses after invasion could have consequences on the invaded ecosystems by modifying the interactions between plants and herbivores and facilitating invasion success. However, no comprehensive biogeographical studies have yet determined the phenotypic levels of plant chemical defenses, as consumed by local herbivores, covering large distributional areas of a species. Senecio pterophorus is a perennial shrub native to Eastern South Africa, expanded into Western South Africa and introduced into Australia and Europe. As other Asteraceae, S. pterophorus contains pyrrolizidine alkaloids (PAs) toxic to vertebrate and invertebrate herbivores.
METHODS AND RESULTS:
Here we analyzed S. pterophorus PAs by LC-MS/MS on foliage sampled across its entire distributional range, including the native and all non-native areas. PA concentrations and diversity was very high: we found 57 compounds belonging to 6 distinct necine base-types, including the highly toxic 1,2-unsaturated PAs (retronecine and otonecines) and the less toxic 1,2-saturated PAs (platynecine and rosmarinecines). Plants from different origins diverged in their PA absolute and relative concentrations. Rosmarinine was the most abundant compound in Australia and South Africa, but it was nearly absent in Europe. We characterized three plant chemotypes: retrorsine-senkirkine chemotype in Eastern South Africa, Rosmarinine chemotype in Australia and Western South Africa, and acetylseneciphylline chemotype in Europe. PA absolute concentrations were highest in Australia.
CONCLUSIONS:
The increased absolute and relative concentrations of retronecine PAs from Australia and Europe, respectively, indicate that S. pterophorus is potentially more toxic in the invasive range than in the native range. | | Carcinogenesis. 1980 Feb;1(2):161-4. | | Evaluation in vitro of several pyrrolizidine alkaloid carcinogens: observations on the essential pyrrolic nucleus.[Pubmed: 22282996] |
METHODS AND RESULTS:
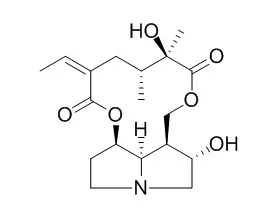
Two hepatocarcinogenic alkaloids (retrorsine and monocrotaline) and one synthetic analogue (synthanecine A bis-N-ethylcarbamate) gave positive results while a non-toxic alkaloid (Rosmarinine) was negative in the test. Positive results were also given by dehydroretronecine, a secondary pyrrolic alkaloid metabolite, and the closely related synthetic compound 2,3-bishydroxymethyl-1-methylpyrrole. These observations lend support to the hypothesis that a simple alkylating pyrrole is the biologically active chemical agent derived from these alkaloids. The negative transformation response observed for the non-carcinogenic alkaloid Rosmarinine establishes that the carcinogenic alkaloids are inducing transformation rather than simply selecting for spontaneous transformants.
CONCLUSIONS:
The mammalian-derived cells used in this study, unlike S. typhimurium, were capable of activating retrorsine in the absence of an auxiliary source of metabolising enzymes (S-9 mix). | | Revista Latinoamericana De Química, 2000 :27-30. | | Insecticidal effect of the alkaloid rosmarinine on Spodoptera frugiperda (J. E. Smith) Lepidoptera: Noctuidae.[Reference: WebLink] |
METHODS AND RESULTS:
Rosmarinine, a pyrrolizidine alkaloid isolated from Senecio callosus stems, leaves and flowers, showed toxic activity against neonate first instar larvae of the fall armyworm Spodoptera frugiperda (maize pest) when tested at 10, 50 and 100 ppm.
CONCLUSIONS:
The alkaloid did not modify significantly the life cycle of S. frugiperda and did not demonstrated antifeedant activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)