| Structure Identification: |
| The Alkaloids: Chemistry and Pharmacology.1989;35:1–76. | | Chapter 1 Alkaloids from Guatteria.[Reference: WebLink] |
METHODS AND RESULTS:
Dehydronornuciferine and O-methyldehydroisopiline are isolated from G. ouregou, where they cooccur with the corresponding noraporphines, and the N-formyl derivatives Dehydroformouregine and formouregine. | | Chinese Traditional & Herbal Drugs, 2015 , 46 (21) :3146-3150. | | Chemical constituents of Sabia parviflora.[Reference: WebLink] | To investigate the chemical constituents from the stems and leaves of Sabia parviflora.
METHODS AND RESULTS:
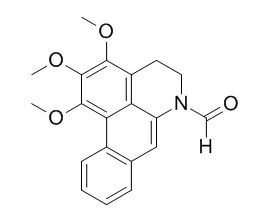
Column chromatographies over silica gel, Sephadex LH-20, reverse phase C_(18), MCI, and semi-preparative HPLC were used repeatedly for separation and purification of chemical constituents, and their structures were identified by NMR and MS spectra data with those reported in literature. Fourteen compounds were obtained from the petroleum ether extract of S. parviflora, and identified as β-sitosterol(1), mominine(2),(20S)-3-oxo-20-hydroxytaraxastane(3), fluoren-9-one(4), N-formyldehydroanonain(5), betulinic acid(6), Dehydroformouregine(7), palmitic acid(8), 20-hydroxy-lupan-3-one(9), 3-oxooleanolic acid(10), erythrodiol(11), methyl betulinate(12), N-formyl-annonain(Z)(13), and N-formyl-O-methylisopiline(14).
CONCLUSIONS:
Among the 14 compounds, there are seven pentacyclic triterpenes, four alkaloids, and three other compounds; compounds 3—10 and 12—14 are isolated from this plant and the plants of Sabia Colebr. for the first time, and the ~(13)C-NMR spectral assignments of compounds 5, 7, and 14 are reported for the first time. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)