| Kinase Assay: |
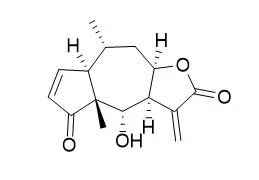
| Cancer Res. 2001 Aug 1;61(15):5817-23. | | Helenalin triggers a CD95 death receptor-independent apoptosis that is not affected by overexpression of Bcl-x(L) or Bcl-2.[Pubmed: 11479221] | Apoptosis is required for proper tissue homeostasis. Defects in apoptosis signaling pathways, thus, contribute to carcinogenesis and chemoresistance. A major goal in chemotherapy is, therefore, to find cytotoxic agents that restore the ability of tumor cells to undergo apoptosis.
METHODS AND RESULTS:
We show here that the sesquiterpene lactone Helenalin (10-50 microM) induces apoptosis in leukemia Jurkat T cells even if they lack the CD95 death receptor or overexpress the antiapoptotic proteins Bcl-x(L) or Bcl-2. Activated peripheral blood mononuclear cells, however, are not affected (10-50 microM Helenalin). Helenalin led to a time-dependent (0-24 h) cleavage of the specific caspase-3-like substrate Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin as well as to the proteolytic processing of procaspase-3 and -8. Caspase activation was a necessary requirement for apoptosis because the broad-spectrum caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk, 50 microM) completely abrogated Helenalin-induced DNA fragmentation as well as phosphatidylserin translocation. Although the initiator caspase-8 was activated, the Helenalin-induced signaling pathway did not require the CD95 death receptor as shown using cells without or with an antibody (ZB4)-blocked CD95 receptor. Helenalin also did not induce CD95 or CD95-ligand expression. On the other hand, Helenalin was found to induce the release of cytochrome c from mitochondria that was not inhibited by the caspase inhibitor zVAD-fmk, which indicated that cytochrome c release precedes caspase activation. Cytochrome c release was accompanied by dissipation of the mitochondrial transmembrane potential (DeltaPsi(m)), which was partly inhibited by zVAD-fmk, which suggests that caspases are involved in loss of DeltaPsi(m). Most importantly, overexpression of the mitochondria protecting proteins Bcl-x(L) or Bcl-2 failed to confer resistance to Helenalin-induced apoptosis, although the data presented here suggest that Helenalin induces a mitochondria-dependent pathway.
CONCLUSIONS:
Thus, Helenalin is a promising experimental cytotoxic agent that possibly points to new strategies to overcome apoptosis resistance attributable to overexpression of antiapoptotic Bcl-2 proteins. | | BMC Complement Altern Med. 2012 Jul 11;12:93. | | NF-κB p65 repression by the sesquiterpene lactone, Helenalin, contributes to the induction of autophagy cell death.[Pubmed: 22784363 ] | To deduce the mechanistic action of Helenalin, cancer cells were treated with the drug at various concentrations and time intervals.
METHODS AND RESULTS:
Using western blot, FACS analysis, overexpression and knockdown studies, cellular signaling pathways were interrogated focusing on apoptosis and autophagy markers. We show here that Helenalin induces sub-G1 arrest, apoptosis, caspase cleavage and increases the levels of the autophagic markers. Suppression of caspase cleavage by the pan caspase inhibitor, Z-VAD-fmk, suppressed induction of LC3-B and Atg12 and reduced autophagic cell death, indicating caspase activity was essential for autophagic cell death induced by Helenalin. Additionally, Helenalin suppressed NF-κB p65 expression in a dose and time dependent manner. Exogenous overexpression of p65 was accompanied by reduced levels of cell death whereas siRNA mediated suppression led to augmented levels of caspase cleavage, autophagic cell death markers and increased cell death.
CONCLUSIONS:
Taken together, these results show that Helenalin mediated autophagic cell death entails inhibition of NF-κB p65, thus providing a promising approach for the treatment of cancers with aberrant activation of the NF-κB pathway. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)