| In vivo: |
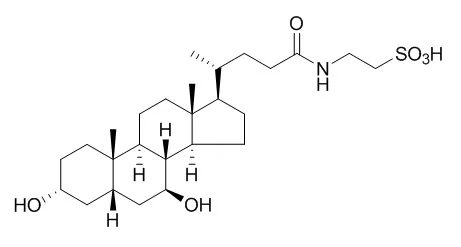
| Diabetes,2010, 59(8):1899-1905. | | Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women.[Pubmed: 20522594 ] | Insulin resistance is commonly associated with obesity. Studies conducted in obese mouse models found that endoplasmic reticulum (ER) stress contributes to insulin resistance, and treatment with Tauroursodeoxycholic acid (TUDCA), a bile acid derivative that acts as a chemical chaperone to enhance protein folding and ameliorate ER stress, increases insulin sensitivity. The purpose of this study was to determine the effect of TUDCA therapy on multiorgan insulin action and metabolic factors associated with insulin resistance in obese men and women.
METHODS AND RESULTS:
Twenty obese subjects ([means +/- SD] aged 48 +/- 11 years, BMI 37 +/- 4 kg/m2) were randomized to 4 weeks of treatment with TUDCA (1,750 mg/day) or placebo. A two-stage hyperinsulinemic-euglycemic clamp procedure in conjunction with stable isotopically labeled tracer infusions and muscle and adipose tissue biopsies were used to evaluate in vivo insulin sensitivity, cellular factors involved in insulin signaling, and cellular markers of ER stress. RESULTS Hepatic and muscle insulin sensitivity increased by approximately 30% (P < 0.05) after treatment with TUDCA but did not change after placebo therapy. In addition, therapy with TUDCA, but not placebo, increased muscle insulin signaling (phosphorylated insulin receptor substrate(Tyr) and Akt(Ser473) levels) (P < 0.05). Markers of ER stress in muscle or adipose tissue did not change after treatment with either TUDCA or placebo.
CONCLUSIONS:
These data demonstrate that TUDCA might be an effective pharmacological approach for treating insulin resistance. Additional studies are needed to evaluate the target cells and mechanisms responsible for this effect. | | Gut. 2008 Oct;57(10):1448-54. | | Tauroursodeoxycholic acid exerts anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent mechanism in rat liver[Pubmed: 18583398 ] | Ursodeoxycholic acid (UDCA) exerts anticholestatic effects in part by protein kinase C (PKC)-dependent mechanisms. Its taurine conjugate, TUDCA, is a cPKC alpha agonist. We tested whether protein kinase A (PKA) might contribute to the anticholestatic action of TUDCA via cooperative cPKC alpha-/PKA-dependent mechanisms in taurolithocholic acid (TLCA)-induced cholestasis.
METHODS AND RESULTS:
In perfused rat liver, bile flow was determined gravimetrically, organic anion secretion spectrophotometrically, lactate dehydrogenase (LDH) release enzymatically, cAMP response-element binding protein (CREB) phosphorylation by immunoblotting, and cAMP by immunoassay. PKC/PKA inhibitors were tested radiochemically. In vitro phosphorylation of the conjugate export pump, Mrp2/Abcc2, was studied in rat hepatocytes and human Hep-G2 hepatoma cells.
In livers treated with TLCA (10 micromol/l)+TUDCA (25 micromol/l), combined inhibition of cPKC by the cPKC-selective inhibitor Gö6976 (100 nmol/l) or the non-selective PKC inhibitor staurosporine (10 nmol/l) and of PKA by H89 (100 nmol/l) reduced bile flow by 36% (p<0.05) and 48% (p<0.01), and secretion of the Mrp2/Abcc2 substrate, 2,4-dinitrophenyl-S-glutathione, by 31% (p<0.05) and 41% (p<0.01), respectively; bile flow was unaffected in control livers or livers treated with TUDCA only or TLCA+taurocholic acid. Inhibition of cPKC or PKA alone did not affect the anticholestatic action of TUDCA. Hepatic cAMP levels and CREB phosphorylation as readout of PKA activity were unaffected by the bile acids tested, suggesting a permissive effect of PKA for the anticholestatic action of TUDCA. Rat and human hepatocellular Mrp2 were phosphorylated by phorbol ester pretreatment and recombinant cPKC alpha, nPKC epsilon, and PKA, respectively, in a staurosporine-sensitive manner.
CONCLUSIONS:
UDCA conjugates exert their anticholestatic action in bile acid-induced cholestasis in part via cooperative post-translational cPKC alpha-/PKA-dependent mechanisms. Hepatocellular Mrp2 may be one target of bile acid-induced kinase activation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)